- Contact Person : Ms. Wang Nancy
- Company Name : Shanxi Jerry Medical Instrument Co., Ltd.
- Tel : 0086-351-7036738
- Fax : 0086-351-7036740
- Address : Shanxi,Taiyuan,Room701/702, Pulse Oximeter, Patient Monitor, Dasheng Tech. Blg., 303Changzhi Road, Taiyuan City, Shanxi Province, China
- Country/Region : China
- Zip : 030006
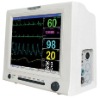
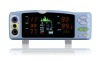
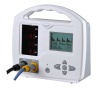
JERRY-A NIBP Patient Monitor
JERRY-A NIBP Patient Monitor
| Two parameters | NIBP, Pulse Rate |
| Waveforms | / |
Features
| 1 | Compact, small, light, easy for carrying and handling |
| 2 | LED and LCD display |
| 3 | Audio and visual alarm with freely set limit |
| 4 | Storage and review of patients data up to 900 groups which is never lost even when powers off |
| 5 | Two kinds of reviewing the data: In list or in single group |
| 6 | Three kinds of power supply, AC Power, Build-in rechargeable Ni/MH battery and Common AA alkaline or rechargeable batteries |
| 7 | Automatic power-off without signal for 10 min |
| 8 | Patients trend can be transmitted to a personal computer for display,saving and printing |
| 9 | Suitable for adult and pediatric |
Applications
NIBP Patient Monitor can be applied in the hospitals operation room, ICU, clinic section office, Out-patient department, sickroom and emergency treatment.
Return Policy
In the event that it becomes necessary to return a unit to the manufacturer, please obtain a return authorization first. Please contact the manufacturer and provide the model number, serial number, and a brief description of the reason for return. Return shipments will not be accepted if the serial number is not clearly visible.
The customer is responsible for freight charges when this product is shipped to the manufacturer for service (including any relevant customs fees or other freight related charges).
JERRY-A NIBP Patient Monitor